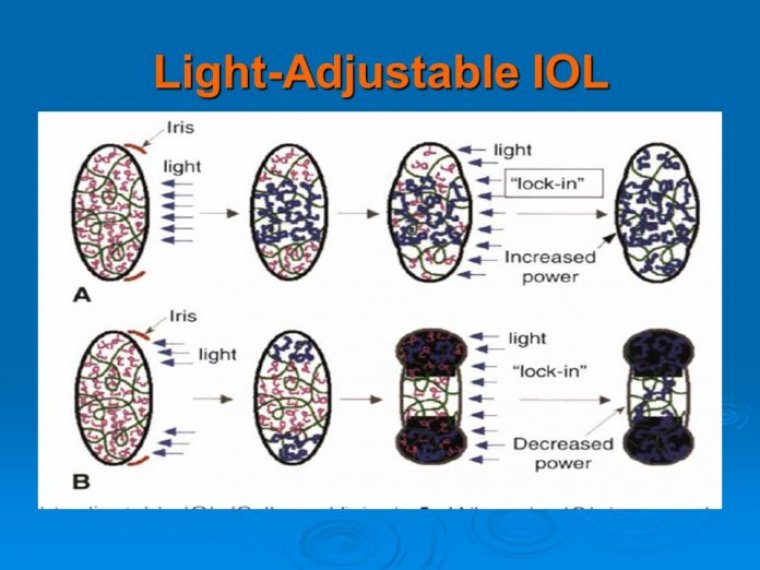
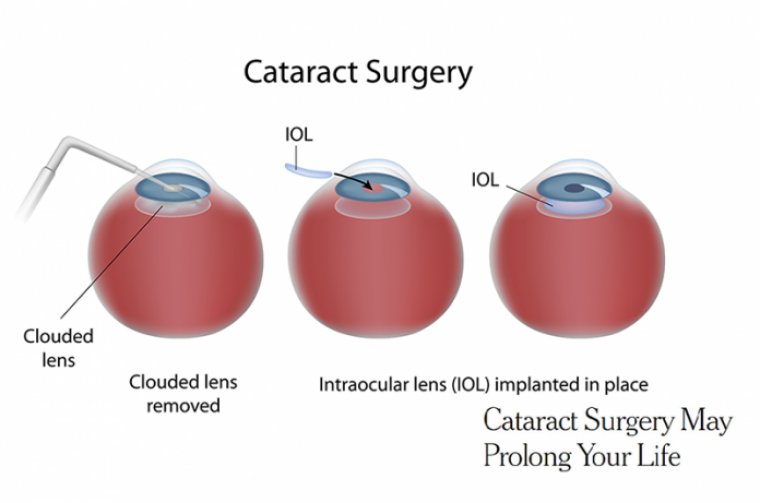
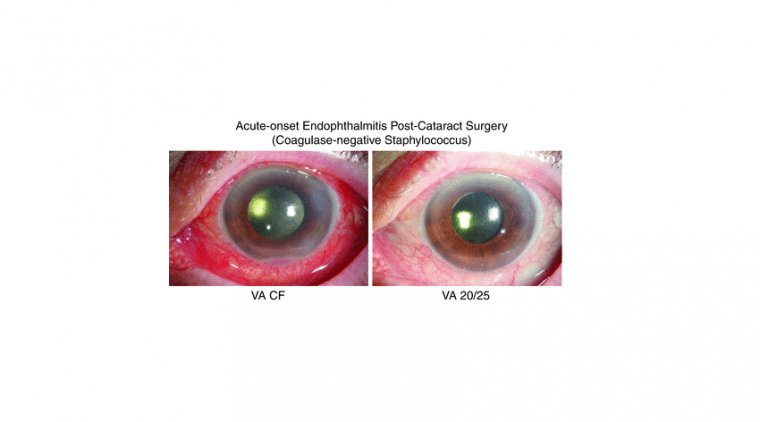
Rayner Announces 1,000 IOLs Recorded on RayPRO Digital Platform
Launched at ASCRS in 2019 for ophthalmic surgeons who perform cataract procedures, RayPRO is a web-based and mobile digital platform that proactively collects an insightful blend of Patient Reported Outcomes (PROs).
Once registered on RayPRO, patients receive five short emailed questionnaires over 3 years that collect feedback on their satisfaction, functional vision and follow-up eye procedures.
The data is then presented to surgeons in real-time via the intuitive RayPRO dashboard, that can be accessed anywhere at any time.
“The amount of data being added to RayPRO demonstrates the value and level of insights our customers derive from the platform.”
“As an example, two hundred records for all our RayOne Trifocal lenses have so far been collected and, at a glance, we can see that patients report 97% spectacle independence–with 98% achieved at distance, 96% intermediate and 94% near,” Mark Vizard, Rayner’s Digital Health Manager, said in a company news release.
“Until now, combining this level of granular insight with a high quantity of patients that is also instantaneously filterable by country was simply not possible. RayPRO continues to go from strength to strength, and we look forward to making data-driven decisions based on feedback from tens of thousands of patients over the coming years.”
Designed for GDPR and HIPAA compliance, Rayner is only able to see metrics at a global or country level and has no access to patient identifiable data.
About Rayner
Since the implantation of the first Rayner intraocular lens by Sir Harold Ridley 1949, Rayner has continuously pioneered intraocular lens (IOL) design with a goal to improve vision and restore sight worldwide.
Today, Rayner’s mission remains to deliver innovative and clinically superior ophthalmic products that respond to the expectations of our global customers to improve the sight and quality of life of their patients.
Headquartered in Worthing, United Kingdom, Rayner markets its IOL, OVD and dry eye portfolio, worldwide in over 80 countries through a network of distributors and includes direct sales teams in the United Kingdom, USA, Germany & Austria, Italy, Spain and Portugal.
A series of recent discoveries harnessing the adaptive immune system of prokaryotes to perform targeted genome editing is having a transformative influence across the biological sciences.
The discovery of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated (Cas) proteins has expanded the applications of genetic research in thousands of laboratories across the globe and is redefining our approach to gene therapy.
Traditional gene therapy has raised some concerns, as its reliance on viral vector delivery of therapeutic transgenes can cause both insertional oncogenesis and immunogenic toxicity.
While viral vectors remain a key delivery vehicle, CRISPR technology provides a relatively simple and efficient alternative for site-specific gene editing, obliviating some concerns raised by traditional gene therapy.
Although it has apparent advantages, CRISPR/Cas9 brings its own set of limitations which must be addressed for safe and efficient clinical translation.
The latest research in the area of clustered regularly interspaced short palindromic repeats (CRISPR) technology may provide a paradigm to accelerate the development of CRISPR–associated protein 9 (CRISPR-Cas9) therapies to correct patient-derived induced pluripotent stem cells in inherited retinal dystrophies, according to Erin Burnight, PhD, associate research scientist in the Department of Ophthalmology and Visual Sciences at the University of Iowa Carver College of Medicine in Iowa City.
Although the ability to select for corrected disease-causing variants is effective in establishing disease models and developing cell therapies for inherited retinal diseases, there is a shortcoming, Burnight said: In some cases, placement of the selection cassette can interfere with the expression from the targeted locus.
In light of this, she and her colleagues theorized that they could address this interference at the intended locus using a cotargeting strategy that would delivering CRISPR-Cas9 reagents to 2 locations.
One targeted the locus of interest; the second, a separate locus. “Thus, this approach produced a cotargeting strategy using the adeno-associated virus vector [AAVS1] safe harbor site as the selection locus,” she said.
How they did it
The investigators reported that they made a plasmid carrying a Streptococcus pyogenes Cas9 expression cassette and single-guide RNA expression cassettes that targeted the locus of interest and the AAVS1 site.
Five sites in total were targeted: CRX, GRK1, VSX2, USH2A, and CEP290. The CRISPR-Cas9 expression plasmid, a donor template carrying homologous sequence to the locus of interest, and a donor template carrying a puromycin selection cassette flanked by AAVS1 homology sequence were delivered to human cells via cationic lipid transfection and cultured under puromycin selection for 10 to 14 days.
Genomic DNA was isolated from puromycin-resistant clones and screened for modification at the locus of interest via polymerase chain reaction.
The investigators successfully cotargeted the 5 sites, and the efficiency of the cotargeting effort varied by locus, Burnight said.
“We cloned and screened single-guide RNAs targeting at the 5 loci. We achieved successful cotargeting at all 5 loci as revealed by amplification of donor-target junctions and RFLP [restriction fragment length polymorphism] analysis,” she said.
“The clonal analysis at CRX, GRK1, and VSX2 showed cotargeting in 5%, 20%, and 35% of puromycin-resistant clones, respectively.” The investigators concluded that their results may provide a paradigm to develop CRISPR-Cas9 treatments for inherited retinal dystrophies.