Intraoperative Aberrometry – Cataract Surgery & IOL Selection
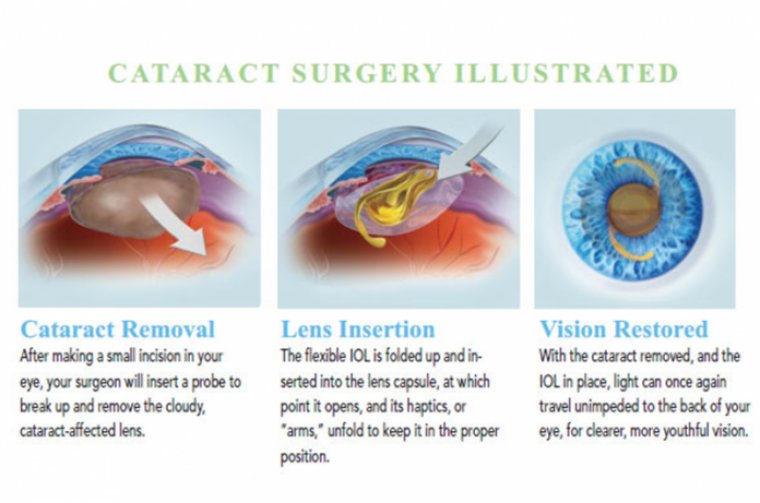
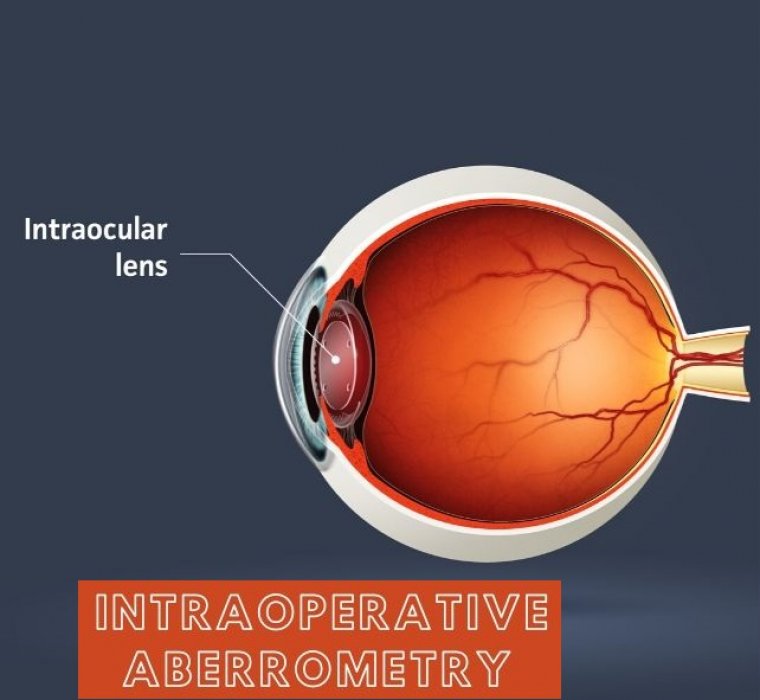
Intraoperative wavefront aberrometry aims to improve refractive outcomes following cataract surgery by optimizing the spherical power of the intraocular lens implant or calculating the appropriate axis and power of toric lenses during cataract surgery in an aphakic state.
Intraoperative aberrometry (IA) is a technique that captures the wavefront and estimates the optical aberrations of eye in aphakic state after cataract removal. It has shown to improve refractive outcomes of cataract surgery, especially in eyes with prior laser vision correction done for myopia.
Intraoperative aberrometry promises to fine-tune cataract surgery results through aphakic refraction.
Innovation in cataract surgery has been abundant in the last several years. New IOL choices, diagnostic tools and intraoperative technologies have expanded postoperative visual possibilities and our offerings to patients.
Visual goals for many patients now include not only an improvement in quality of vision but also some degree of spectacle independence.
With technological advances, we have become more equipped to help our patients achieve these evolving goals and meet or exceed expectations, which requires precision in the selection of IOLs, including monofocal, toric and presbyopia-correcting options.
Intraoperative aberrometry (IA) provides an opportunity to increase available data for IOL power determination beyond biometry and other preoperative measurements alone.
With the development of more sophisticated biometers and topographers that measure both anterior and posterior cornea and modern IOL formulas, our ability to minimize postoperative refractive error has improved.
While IA has been available for more than a decade, understanding what it offers, its limitations and the current literature are key to optimizing the application of this unique tool.
INCORPORATING THE TECHNOLOGY
IA was introduced commercially in 2009 with Optiwave Refractive Analysis (ORA, Alcon). First, preoperative data is added into an online, cloud-based system.
Intraoperatively, through the use of Talbot-Moiré interferometry, the refraction of the optical system is measured in its current, real-time, intraoperative state. Measurements are typically first taken in the aphakic state, after cataract and cortical removal.
This initial reading gives the refractive error of the system at that time point and displays estimated residual refractive error for a given preselected IOL type and power. This helps refine IOL selection prior to implantation.
Intraoperative Aberrometry can be repeated following IOL implantation to confirm power selection and, in cases of toric lenses, IOL axis positioning. The system incorporates image guidance projected into the field of view for surgical navigation and lens positioning.
Measurements can be affected by wound hydration, epithelial irregularity from surface dryness, viscous dilating gel or viscoelastic on the epithelium, lid speculum positioning and patient fixation.
Careful attention should be given to the ocular surface preoperatively and intraoperatively to prevent drying of the epithelium and subsequent reduction in the quality of the IA measurements.
Close observation of the above variables that can influence readings must be maintained, and care should be taken to avoid basing IOL selection decisions on unrepresentative data.
COST
IA is an elective refractive service and thus not covered by insurance. The cost can be itemized or bundled with a refractive package with astigmatism or presbyopia management.
Outof-pocket responsibility and agreement are often formally documented with an Advanced Beneficiary Notice of Noncoverage, though billing procedures may vary.
Cost of the procedure includes the up-front cost of the device itself, which includes an integrated microscope mount and a portable screen for measurement visualization, and a fee per use or a “click fee.”
Contracts vary, but use of advanced technology lenses from the same manufacturer as the device may result in a waved or reduced price-per-use fee.
UTILITY OF IA
Some studies show increased accuracy in achieving desired refractive outcomes with the use of IA in routine cases, though others show similar results when comparing postoperative refractive error with the use of IA compared to rigorous IOL calculations with modern biometry.
The difference in outcomes of these studies may lie in the specific formulas utilized for IOL power calculation, with newer generation formulas such as Barrett Universal II and Hill-RBF tending to outperform older formulas.
IA is practically the most valuable in cases with variable or ambiguous preoperative measurements where selection of an IOL power or toricity proves challenging. This includes cases where different IOL formulas call for varying IOL powers for a given refractive goal or in eyes with short or long axial lengths.
IA offers one more data point that can be considered in conjunction with other available data to make the most well-supported lens selection.
PRIOR CORNEAL REFRACTIVE SURGERY
IA can also be useful in patients where the prediction error of postoperative refractive outcome has been shown to be greater than in eyes that fall within the range of normal. Of particular interest are post-corneal refractive surgery cases.
Because of the surgically reshaped anterior cornea, many traditional IOL power calculation methods are rendered inaccurate.
Despite the use of multiple online resources that have been developed to determine IOL power in these cases, such as those compiled on the ASCRS postrefractive IOL calculator, these patients are known to have higher error in postoperative refractive results.
Newer technology in biometry and formula development, such as utilization of total corneal power instead of anterior keratometry, have not shown to be of consistent benefit in eyes postkeratorefractive surgery.
The increased error in this post-refractive surgery group is especially challenging, because these patients have often been accustomed to and highly value spectacle independence.
Though studies have shown mixed reports on whether IA consistently improves refractive results in these patients, IA does provide one more piece of data to incorporate into the whole clinical picture to hone IOL selection.
In patients who have previously undergone radial keratotomy (RK), data acquisition with IA may be difficult or impossible. IA has been used successfully in some cases, though prediction error may be worse than for LASIK or PRK.
Hyperopic refractive results with the use of ORA in RK patients have been documented and prediction errors has been shown to rise with increasing number of RK cuts.
Variability may be due to difficulty with consistent data acquisition or intraoperative fluctuations in corneal shape due to the RK incisions. So, while IA may be beneficial in post-RK eyes with few corneal cuts, caution should be taken in the interpretation of data in eyes with multiple cuts or significant corneal opacity.
REFRACTIVE SURPRISES
IA is beneficial in eyes that are outliers. Because of anatomical differences in effective lens position and other variables that result in error in IOL power calculation, some eyes end up undesirably myopic or hyperopic despite the careful use of advanced formulas and rigorous acquisition and interpretation of biometry.
Specifically, IA can be invaluable in IOL selection for second-eye surgeries of patients who had a refractive surprise in their first eye.
Combined with meticulous analysis of the quality of the biometry and consideration of the postoperative refractive result from the first eye, IA can provide supplemental data for determining IOL selection in the second eye or during IOL exchange in the first eye if indicated.
The extra real-time refractive data gives assurance to both the surgeon and the patient that extra measures with all available technologies are being utilized to optimize the outcome.
ADVANCED TECHNOLOGY LENSES
IA can be a valuable tool in cases where astigmatism correction is planned. Though online astigmatism calculators and nomograms have become more sophisticated, calculations are still dependent on measurements taken preoperatively, often relying on assumptions of the magnitude and direction of posterior corneal astigmatism instead of direct measurement of the optical system.
The use of IA has been shown to optimize astigmatism correction with limbal relaxing incisions at the time of cataract surgery, potentially reducing the need for refractive touch-ups postoperatively.
IA can also help in decisions of magnitude and direction of astigmatic correction with toric lenses as well as guide lens positioning at the appropriate axis. With toric placement, an aphakic measurement is often first taken to determine the sphere and cylinder power of the implant lens.
Projected alignment markers guide axis positioning, and repeat measurements following IOL insertion and positioning determine the residual refractive error of the system as well as recommend rotation of the lens if needed to achieve maximal reduction of astigmatism.
In addition, utilizing IA when implanting presbyopia-correcting lenses supplies data from an additional modality to increase confidence in lens power selection and potentially avoid the need for more invasive procedures to enhance refractive results.
Multifocal, extended depth of focus and trifocal lenses have low tolerance to residual refractive error.
Even low magnitudes of myopia, hyperopia or astigmatism result in significant image degradation and patient dissatisfaction with these lenses, often necessitating lens exchange, rotation or corneal refractive touch-up to achieve satisfactory results.
DIFFICULT CASES
In some cases, data from IA may be desirable but unattainable due to anatomical or iatrogenic factors. When considering IA, it is important to note preexisting conditions that may limit data acquisition and discuss with the patient preoperatively.
Some common conditions that may limit image capture and measurement include keratoconus, central corneal opacity, anterior basement membrane dystrophy, Fuch’s endothelial corneal dystrophy and asteroid hyalosis.
While measurements can be attempted in these cases, results should be interpreted with caution. As available technology for IOL choices increases, our patients’ expectations and tolerance to refractive error decreases.
More sophisticated optical biometers, newer generation formulas for power calculation, adjustable lenses and IA are just some of the tools we have available to improve precision and optimize refractive outcomes.
Though the sophistication of our tools has increased substantially over recent years, some cases still pose challenges, particularly those in patients who have undergone previous corneal refractive surgery or in patients who had an unexpected refractive result in their first eye.
Utilization of all available technology helps us offer the most comprehensive and sophisticated approach to deliver predictable outcomes and help patients achieve their advancing visual goals.