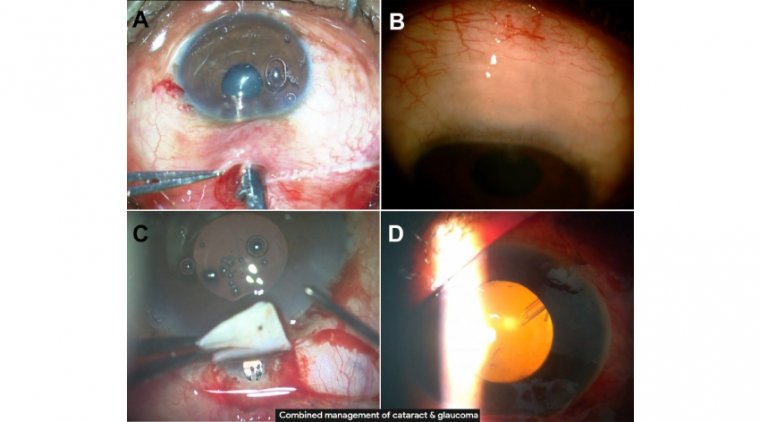
Cataracts & Floppy Iris Syndrome
These complications can be avoided when high-risk patients are identified by preoperative screening and appropriate measures are used intraoperatively.
Surgeons should be prepared to employ a range of perioperative interventions in a graded response to IFIS of different severities.
- Billowing of a flaccid iris stroma in response to ordinary intraocular fluid currents;
- Propensity for iris prolapse toward the phaco and/or the side-port incisions;
- Progressive pupil constriction.
A grading system for IFIS was suggested based on the presence of the previously described intraoperative signs as follows: no IFIS (stable iris without significant miosis), mild IFIS (noticeable floppy iris only), moderate IFIS (floppy iris and miosis) and severe IFIS (floppy iris, significant miosis and strong tendency to iris prolapse).
Two additional pupil features may be present in IFIS:
In contrast to other causes of small dilated pupil and progressive intraoperative miosis (e.g. diabetes), IFIS is characterized by an elastic iris which does not dilate with mechanical stretching.
Therefore, it is important to anticipate IFIS and use pupil expansion devices as a preventive measure in the beginning of surgery rather than after IFIS has developed and the capsulorrhexis is already completed (which might compromise its integrity).
Strategies for the management and prevention of floppy iris syndrome include intraoperative interventions and recommendations on alternatives to tamsulosin for medical therapy in men with cataracts.
The first report associating use of the α1A antagonist tamsulosin with intraoperative floppy iris syndrome (IFIS) appeared in the literature in 2005.
Subsequently, studies have examined the underlying mechanism for the complication and determined that it occurs in women as well as in men, with other α1-antagonists, when α1-antagonist treatment has been stopped preoperatively, and in patients without a history of taking an α1-antagonist.
Furthermore, research shows that a significant proportion of physicians who prescribe
α1-antagonists fail to consider or are even unaware of how these medications can cause complications during cataract surgery.
With this background in mind, cataract surgeons need to carefully review medication history as part of the preoperative evaluation so that they can identify at-risk patients and be knowledgeable about the strategies for IFIS management.
In a clinical update on IFIS during the American Academy of Ophthalmology’s 2020 virtual meeting, Dr David F. Chang reviewed the literature on these topics and provided some practical advice.
Dr Chang works in private practice in Los Altos, California, United States, and is a clinical professor of ophthalmology at the University of California, San Francisco, US.
He co-authored the 2005 paper linking tamsulosin use with IFIS and has since been involved in research and education about this complication.
Insights Into IFIS Risk
Dr Chang presented information on the pharmacology and pharmacokinetics of drugs that block α1-adrenergic receptors to explain why, within this medication class, tamsulosin is associated with the greatest risk of IFIS.
In addition, he discussed evidence showing that tamsulosin not only blocks the α1A receptor in the iris dilator muscle but also causes atrophy of the dilator muscle, to explain why discontinuing treatment preoperatively does not eliminate IFIS risk.
There are potential factors to consider and investigators have looked at these issues. Dr Chang reviewed studies that identified other possible risk factors for IFIS, including hypertension and reports of IFIS in patients with no history of α1-antagonist use.
Among the latter papers was a prospective study co-authored by Dr Chang that documented that in the absence of any epinephrine in the irrigating bottle, severe IFIS occurred during cataract surgery in almost 5% of patients who had no history of α1-antagonist use.
He added that the study was also among the first to provide evidence supporting a benefit for routinely adding epinephrine in the irrigation bottle to prevent mild to moderate IFIS.
“The commercially available fixed combination of phenylephrine 1%/ketorolac 0.3% (Omidria, Omeros Corp.) is approved for maintaining pupil mydriasis, but off-label use of epinephrine in the bottle is also very effective,” Dr Chang said.
Prevention & Management
As an alternative to adding phenylephrine 1%/ketorolac or epinephrine in the irrigation bottle, Dr Chang recommends considering direct intracameral injection of an α-agonist, such as epinephrine or phenylephrine, for patients with a history of α1A-antagonist use.
“Because of its low pH, epinephrine should be first diluted 1:4000 or 1:5000 with BSS [balanced salt solution] prior to off-label intracameral injection,” Dr Chang said.
Epinephrine formulations containing chlorobutanol preservative or tartaric acid are not intended for intraocular use and should be avoided.
Unpreserved epinephrine ampules may contain bisulfite 0.1% to slow oxidation, but corneal endothelial toxicity is avoided with the recommended dilution.
Intracameral injection of phenylephrine 1.5% offers an effective alternative to epinephrine for preventing IFIS, however, this must be obtained from a compounding pharmacy in the United States.
For any compounding of intraocular drugs, Dr Chang recommended using a 503B outsourcing facility that is authorised to ship products nationally and is subject to stringent Food and Drug Administration regulations and inspection.
Dr Chang cautioned against doing pupil stretching to manage IFIS or a small pupil in a patient at risk for IFIS but said iris retractors and mechanical pupil expansion rings are very effective for maintaining an enlarged pupil.
“I use a pupil expander if there is any question about severe IFIS risk and also when there is a comorbidity, such as pseudoexfoliation, a dense lens or perhaps a shallow chamber, as well as when the pupil is not dilating well, even with intraocular phenylephrine,” Dr Chang said.
Strategies for preventing IFIS also include efforts to educate physicians who prescribe α1-antagonists to treat benign prostatic hyperplasia (BPH) about the risk.
These prescribers should be counselled to ask patients if they have cataracts, consider advising patients with symptomatic cataracts to undergo cataract surgery prior to initiating nonemergent treatment with an α1-antagonist, or treat BPH with a medication other than tamsulosin that carries a lower risk of IFIS or no risk.
Dr Chang said he recommends that prescribing physicians consider tadalafil used alone or in combination with dutasteride as a first-line treatment for BPH in their patients with cataracts.
While it is better known as a treatment for erectile dysfunction, tadalafil is a phosphodiesterase-5 inhibitor that also can offer some benefits for patients with lower urinary tract BPH symptoms by improving bladder emptying.
According to Dr Chang, dutasteride, a 5α-reductase inhibitor, decreases prostate size and offers an option for patients. It can prove to be a viable option for ophthalmologists. When an α-antagonist is needed, Dr Chang noted that alfuzosin carries a low risk of postural hypotension in patients and is less likely to cause severe IFIS than tamsulosin, according to a masked comparison study he and his colleagues published.