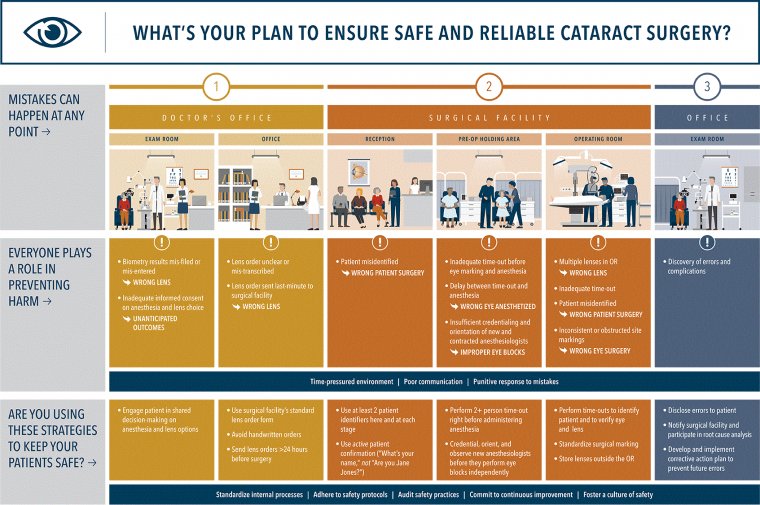
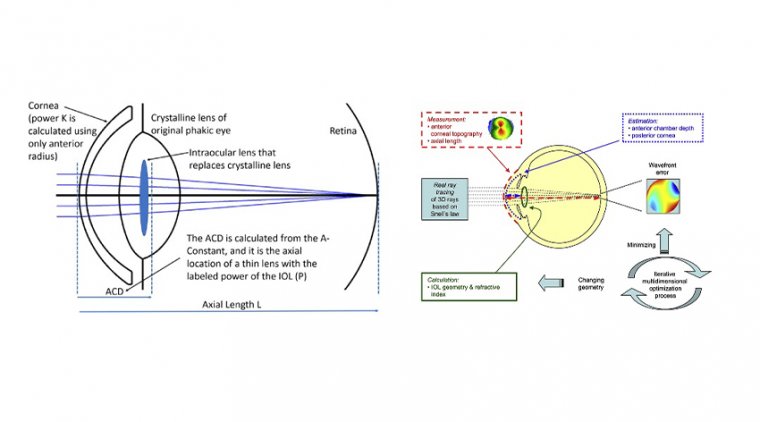
Johnson & Johnson Vision – New Comparative Clinical Study Findings & TearScience LipiFlow Treatment In Cataract Surgery
Patients that received thermal pulsation treatment prior to cataract surgery reported halos significantly less than control group (58.6 vs. 79 percent; p = 0.019)
The study is one of 21 company-sponsored studies being presented the 2021 ASCRS∙ ASOA Annual Meeting
Johnson & Johnson Vision, a global leader in eye health and part of the Johnson & Johnson Medical Devices Companies, revealed new comparative clinical study findings evaluating TearScience LipiFlow treatment in cataract surgery.
The data concluded that treating patients with TearScience LipiFlow prior to cataract surgery significantly reduced patient-reports of halos compared to control group (58.6 vs. 79 percent) and showed larger improvements in Meibomian Gland Score (MGD) than control group (107 vs. 66 percent).
The results were shared today in a poster presentation at the American Society of Cataract and Refractive Surgery (ASCRS) and American Society of Ophthalmic Administrators (ASOA) Annual Meeting.
The study titled, “Preoperative Treatment of Meibomian Gland Dysfunction with Thermal Pulsation System Prior to Extended Depth of Focus IOL Implantation,” was a post-market, prospective, randomized, multi center, bilateral, open-label, cross-over, comparative clinical study covering five U.S. sites and comprising 115 subjects.
At three-months following surgery, there were notable differences in the rates of bothersome ocular symptoms, such as seeing halos, between the study and control groups. The findings align with the recent ASCRS clinical guidelines recommending the proactive diagnosis and management of MGD in the preoperative cataract patient.
“We continue to learn more about the connection between the health of the ocular surface and visual outcomes following cataract surgery,” said Daniel Chang, MD. “This new data reinforces the importance of proactively screening for and treating MGD prior to cataract surgery. It’s a simple addition to the patient’s treatment plan that may result in better visual outcomes for our patients.”
MGD is a prevalent, chronic, and progressive disease that becomes worse the longer it goes untreated. In the study, presurgical TearScience LipiFlow in cataract patients improved meibomian gland function and improved postoperative anterior ocular health.
In addition to reducing postoperative reports of halos, the presurgical treatment also showed measurable improvements in visual acuity (20/20 at 4m, 20/20 at 66cm; 20/30 at 40cm).
“As clinicians, we always want to optimize surgical outcomes for cataract patients, including postoperative visits.
The data we unveiled today reinforces the need for surgeons to screen for, and treat, MGD prior-to cataract surgery, especially given the high incidence and asymptomatic nature of ocular surface disease in patients presenting for cataract surgery,” said Rajesh Rajpal, MD, Chief Medical Officer and Global Head of Clinical and Medical Affairs at Johnson & Johnson Vision.
The study is available for on-demand viewing in the Education Hub at the convention center during the meeting, and remotely on the ASCRS website post-meeting.
About TearScience LipiFlow
The TearScience LipiFlow Thermal Pulsation System, is a medical device used by physicians in addressing Meibomian Gland Dysfunction (MGD).
It consists of a Console and a single-use sterile device, known as the Activator, and has a drug-free mechanism of action. Eye care professionals use the TearScience LipiFlow System to treat MGD patients in-office with confidence and efficiency.