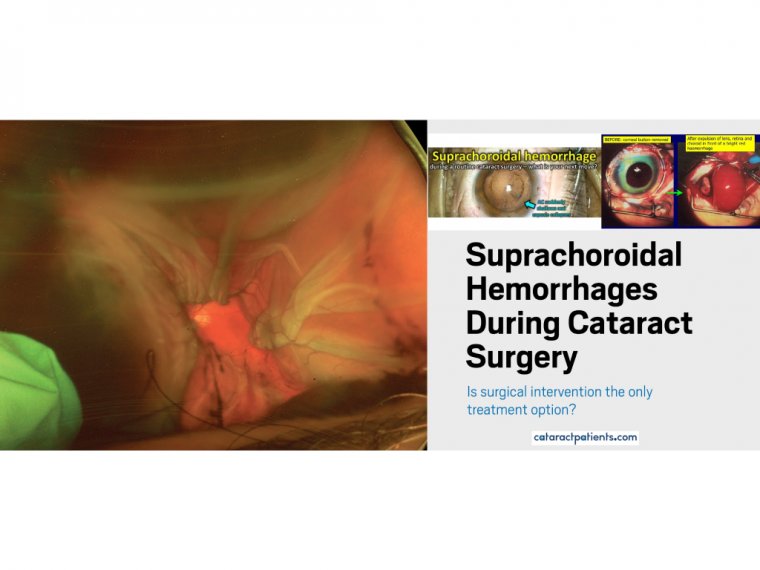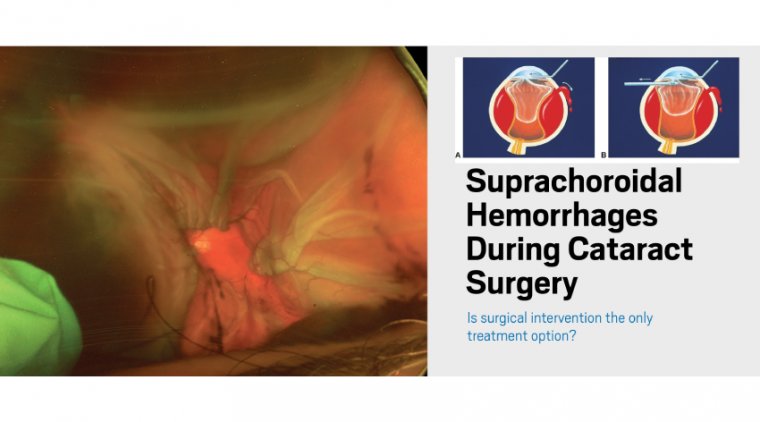
Understanding the Benefits of Laser Cataract Surgery
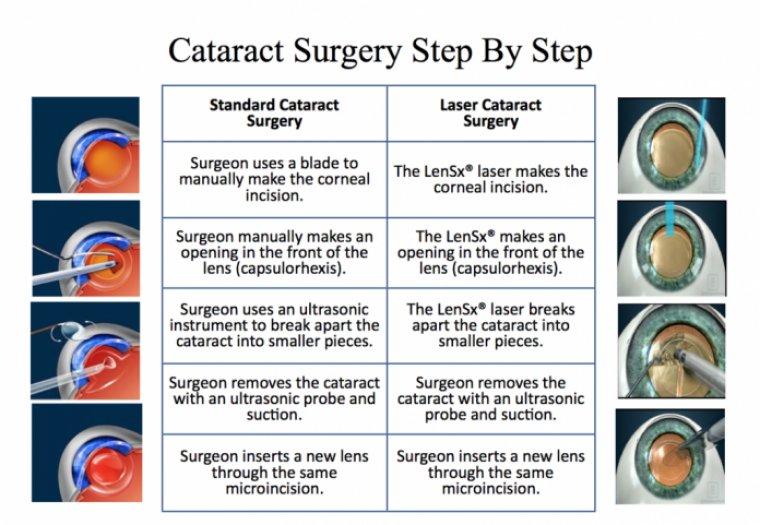
In laser cataract surgery, a laser is used to make the incision and lens opening and to fragment the lens.
It creates a more reliable, repeatable, precise incision than a surgeon can do by hand. Even a very good surgeon can’t make a perfectly circular opening as precisely as a laser can.”
The laser is particularly useful when a cataract is dense or the opening is difficult to create. However, the laser’s accuracy is also important when surgeons want to implant a multifocal lens, which corrects distance and close-up vision, or a toric lens that corrects astigmatism.
There are three steps within cataract surgery in which laser technology can offer a superior technique to the use of conventional hand-held tools.
The Corneal Incision
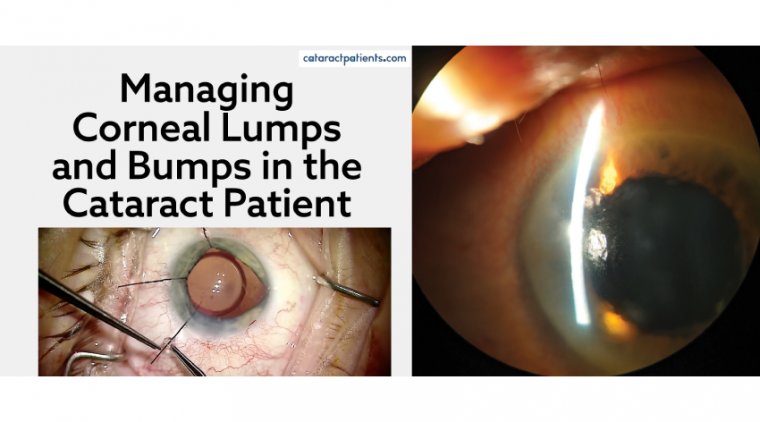
During cataract surgery, doctors make an incision into the cornea in order to access, break up, and then completely remove the clouded lens.
In conventional surgery this involves using a small, hand-held blade that relies entirely on the steadiness of the Ophthalmologist’s hand, accuracy, and experience. The incision is performed in such a way that it will quickly heal without the need for sutures.
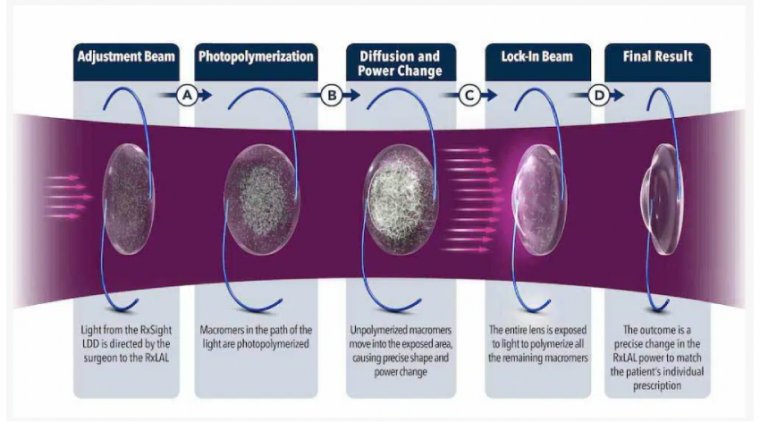
In laser cataract surgery, this part of the procedure is fulfilled using advanced laser technology. The surgical plan is created with a highly sophisticated 3D image of the eye known as an OCT or Optical Coherence Tomography.
During laser cataract surgery, the chance of human error is greatly reduced, which improves the likelihood that the incision will be in the correct spot and will heal more effectively with minimal risk of infection.
The Capsulotomy
In order to access the cataract, it is first necessary to remove a section of the thin, clear capsule that protects it – a process known as a capsulotomy. It is essential that this is done carefully so that the rest of the capsule remains intact, since it will not regrow.
This portion of the cataract surgery is important for ensuring adequate support of the artificial lens implant (IOL) that will be replacing your cloudy natural lens.
During conventional cataract surgery, this is done by hand using a small needle and tiny forceps, but the results of this technique can be unpredictable in unexperienced hands.
However, by swapping the manual tools for laser technology, we can create the access to the cataract with far greater precision and accuracy.
This is also beneficial when it comes to inserting the artificial intraocular replacement lens. This is because research indicates that capsulotomies that are performed with laser technology enable better positioning of the intraocular lens, which in turn can improve the overall visual outcome from your procedure.
Fragmentation of the Lens and Cataract
To be able to successfully remove the cloudy lens, doctors first have to fragment the damaged lens. This means it needs to be broken up into lots of much smaller pieces.
During traditional cataract surgery, this is done using an ultrasonic device that is inserted through the incision. This process, known as phacoemulsification, can cause the inside of the eye to rapidly heat up, putting the patient at serious risk of experiencing a burn in and around the incision.
This can cause several complications, from making it difficult for the incision to self-seal to changing the shape of the corneal tissue and inducing astigmatism.
However, when a laser is used to fragment the cataract it also makes the pieces it creates much softer. This means that less energy is needed to remove them, and this lowers the risk of experiencing burning and subsequent complications.
Reducing the amount of energy used can also help better preserve the capsule for the IOL to sit in. It also lowers your risk of other ocular complications such as a detached retina.
Past & Future
For over 40 years since the advent of small-incision phaco by Dr. Charles Kelman, many of the important aspects of cataract surgery have been performed manually — hampered by the possibility of imprecision and rare occasional complications.
Our initial incision into the eye through the clear cornea serves as the foundation on which to build the rest of the surgical procedure and requires difficult maneuvers and control. As refractive cataract surgeons, we want to control the placement, architecture, size and shape of our cataract incisions to create the desired astigmatic effect and optimal wound construction for healing.
Additionally, we maintain intraoperative and postoperative anterior chamber pressure through our clear corneal incisions and reduce risk of endophthalmitis by creating tightly sealed wounds, ideally watertight and self sealing without the need for sutures. Amazingly, we accomplish this in the majority of cases with simple keratomes of various materials and sizes.
Clearly, creating a clean, centered capsulorhexis is the key to successful cataract surgery. An errant capsulorhexis increases the subsequent surgical difficulty and risks for complications.
As it is presently performed manually, the capsulorhexis is hard to size and difficult to center due to parallax error. In addition to traditional capsulorhexis sizing, novel IOLs have presented additional requirements to prevent posterior capsule opacification, tilt and decentration.
As surgeons, we have to estimate the capsulorhexis position and size so that the desired aperture in the capsular bag after healing, with possible fibrosis, matches the design objective of the intraocular lens.
For square-edged IOLs, the capsulorhexis is sized for optimal overlap but for some of the accommodating IOLs such as the Crystalens, the capsulorhexis is oversized to allow for hinged movement.
Additionally, placement of the capsulorhexis is thought to aid IOL centration and yet we have few tools to help us center the capsulorhexis besides anatomical landmarks such as the dilated pupil or limbal edge.
This can be challenging given irregular dilation and pharmacologically induced inferotemporal pupil dilation prior to phaco surgery. Decentration of multifocal lenses is debilitating for our patients, causing double vision, glare, halos, etc.
Our current tools consist of cystotomes and forceps used to manually tear the capsulorhexis and a variety of aid devices for sizing, such as capsulorhexis rings, markers and the Seibel ruler.
If you add the surgical challenges of floppy irises, shallow chambers and zonular weaknesses, the capsulorhexis becomes increasingly difficult, even for the experienced surgeon.
During phacoemulsification, we rely today on a variety of cracking and chopping techniques to disassemble the lens nucleus while maintaining the structural integrity of the capsular bag and supporting zonules.
Perhaps the sheer number of surgical phacoemulsification techniques for soft lenses, hard lenses and complicated cases indicate that there is still room for improvement.
The number and degree of instrument manipulations required to disassemble the lens, remove the cortex and polish the capsular bag poses additional risk of corneal phaco burn, iris and endothelial damage and reduced integrity of wound size and architecture.
As refractive cataract surgeons, our goal is to provide minimal astigmatism required for optimal uncorrected vision, a target of less than 0.75 D of residual astigmatism after surgery.
While cataract surgery is safe and dependable today, there is still much room for improvement. Increased precision and safety will improve the results of both standard and premium IOL surgery.
Perioperative and Postoperative Safety and Performance
When we think about the possibilities of femtosecond laser-assisted cataract surgery, we think it is a tool that levels the surgical playing field by facilitating simplicity and reproducibility and also enables future innovation from our best and brightest surgeons.
We are not just replacing manual methods with a more precise and controlled technique, but are also creating the ability to cut with architectural and locational control not possible with manual methods, keratomes, cystatomes, forceps, choppers and phaco tips.
The femtosecond laser enables precise cuts that have important safety and performance implications both peri-operatively and long term. Integrated imaging systems, such as on-board OCT, provide registration to direct the laser both laterally and in depth.
For clear corneal incisions, the OCT can provide the corneal thickness at the desired incision location so the architecture for incisions can be properly customized for each patient.
This can include the main cataract incision sized for surgical instrumentation, such as the phaco tip and lens injector, and is designed for a watertight seal. It can also include side port incisions.
The depth and architecture for relaxing incisions can also be registered, calculated and accurately delivered on the desired axis.
For the capsulorhexis, the OCT detects the iris boundaries so the laser can be safely directed inside the iris even if it is not dilated symmetrically. Research has shown that the capsular edge tensile strength is equal to that of manual techniques.
The laser incision formed with cavitation bubbles may have improved strength and resistance to manual trauma during phaco and lens implantation, which could reduce capsular extensions.
Symmetrical uniform fibrosis and healing may also be important for accommodating IOLs that rely on symmetrical contractile forces to translate into axial movement.
The OCT also provides improved references that enable algorithms to calculate desired capsulotomy centration, reducing surgeons' reliance on visible reference points and estimation for size, shape and position.
With this registration information, the laser and control system can deliver precisely sized, custom-shaped and calculated centration of the capsule aperture.
Finally, the OCT detects the anterior and posterior surfaces of the capsule so customized laser patterns can be delivered to separate the lens.
Detection of the posterior surface is critical to maintain a safety zone and prevent laser cuts in the posterior capsule, which could lead to rupture during phaco.
The nuclear segmentation can be customized for the lens density to facilitate separation with traditional instruments such as simple spatulas without the need for sculpting or chopping.
Additional patterns can soften the lens and match to preferred phaco techniques and lumen size so overall phaco time and energy can be reduced.
Patterns can be optimized for followability for phaco dynamics to reduce flow, trampolining, iris prolapse and endothelial cell damage from lens fragments.
We have found that quadrant segmentation with a cube-softening pattern can turn a LOCS III grade 4 nucleus into that which resembles irrigation and aspiration needs for a grade 2 nuclear cataract.
There may be added safety benefits with less instrument manipulation from fewer instruments and fewer insertion and removal motions.
The integration of advanced imaging technology and customized algorithms with femtosecond laser pulse cutting precision in the cornea, capsule and cataractous crystalline lens provides a new level of safety and security for the cataract surgeon.
Adoption Challenges
We see a few challenges for adoption of femtosecond technology for cataract surgery, all of which are encompassed in workflow and practice integration.
While femtosecond lasers at customer-centered private outpatient refractive centers were afforded private funding, space and time, we will need to integrate this technology into crowded, CMS-regulated, ambulatory surgery centers that are driven by cost and efficiency.
Femtosecond technology is expensive and, for the safety and control necessary for cataract surgery, we will also be paying for integrated imaging systems.
I believe the procedural cost will initially be absorbed in the premium lens and refractive channel The challenge will be in finding a way to pay for the advantages of this technology for all lens implants, both standard monofocals and toric, multifocal and novel lenses.
We do not expect that overall time in the operating room will be reduced for highly efficient centers and surgeons. However, a surgeon who currently spends 30 to 45 minutes on a case may realize significant savings in time.
Given the procedural time and technology expense, how do we seamlessly bring this laser into our operating rooms? The four pieces to this puzzle will be the number of operating rooms at your facility, the number of laser systems available, the number of surgeons operating at the same time and the number of operating rooms used by a given surgeon.
We will want to optimize the workflow so the laser system can be used by all operating surgeons to pre-treat the maximum number of patients without multiple large capital equipment expenses.
Another key difference from refractive surgery in workflow integration is the cataract patient population. The aging population with comorbidities requires more attention to safety and patient comfort.
Additionally, we have the challenge, and therefore the opportunity, to create a premium surgical experience for these patients.
As a surgical community, we will need to find creative solutions to elevate the safety and results of refractive cataract surgery A good first step is to figure out how to integrate femtosecond technology into our cost and efficiency-driven workflow.
Femtosecond Laser for Refractive Cataract Surgery: Equalizer or Enabler?
Femtosecond laser-assisted cataract surgery is a promising improvement on standard contemporary phacoemulsification cataract surgery It has the potential advantages of improved safety increased precisions and enhanced reproducibility.
What does this mean for us, the surgeons, our practices and ASCs, and our patients? Will this technology obsolesce the years of experience that we have acquired? Will the challenges of modern refractive and cataract surgery still attract the best and the brightest?
Femtosecond lasers offer the opportunity to bring a surgeon's talent and expertise to the forefront of every surgery, less hampered by imperfect surgical tools. In an age in which cataract surgeons cannot keep up with the growing aging population around the world, this technology holds promise of better vision for those with limited access to world-class surgeons.
More importantly, this technology will enable innovation from the surgical community and our partners in industry, particularly those in the lens design and manufacturing business.
We will soon have a tool that will allow us to create controlled relaxing incisions sub-Bowman's layer, complex cataract incisions, customized capsulotomies, capsulotomies integrating with IOLs such as “Capsule in the Lens,” new methods for toric IOL orientation and even lens refilling surgery.
Our goal is to provide a tool that facilitates this future innovation and improves precision and reproducibility today for all stages of the surgical process from cataract removal to IOL implantation and wound healing.
Through enabling IOL performance, and reducing surgical complications, we will increase patient satisfaction through emmetropic refractive outcomes that improve quality of life.