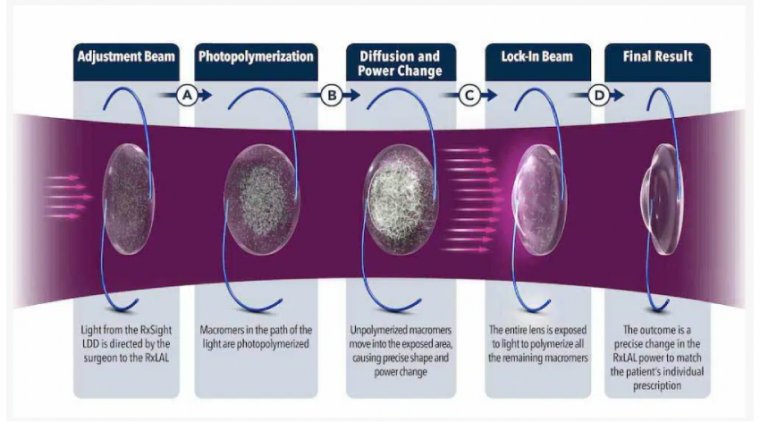
Metrovision Receives FDA Clearance for MonPackONE for Vision Electrophysiology
Metrovision Receives FDA Clearance for MonPackONE for Vision Electrophysiology
PÉRENCHIES, France – Metrovision SAS, received FDA clearance for its MonPackONE Vision Monitor for vision electrophysiology applications including ganzfeld flash ERG and VEP, pattern ERG and VEP, multifocal ERG and VEP as well as sensory EOG.
The MonPackONE is compatible with the ISCEV standards for vision electrophysiology. Thanks to its modular design, it can be configured to suit individual needs and is easily upgradeable.
MonPackONE can be combined with MonCvONE-CR, creating the most versatile platform for visual function analysis in the world.
It is the only commercialized instrument able to perform both dark-adapted and light-adapted full-field perimetry. Other capabilities include dark adaptometry, FST, pupillometry and vision electrophysiology testing.
“For years Metrovision has been a leader in vision electrophysiology with units in over 30 countries. We are pleased to be able to offer this advanced and modular technology to researchers and clinicians in the United States.” Says Jacques Charlier, PhD, CEO.
Founded 30 years ago, Metrovision develops, manufactures and markets specific solutions for measuring visual functions. In the USA, Medical Device Success distributes the Metrovision line of products.