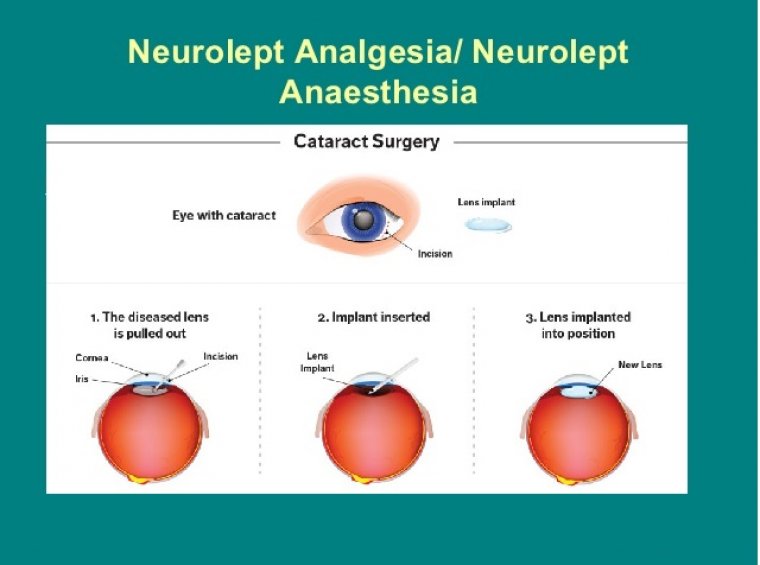
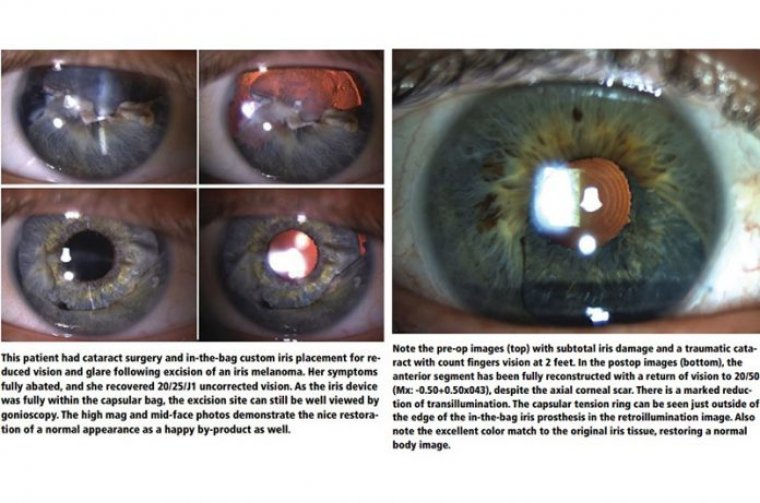
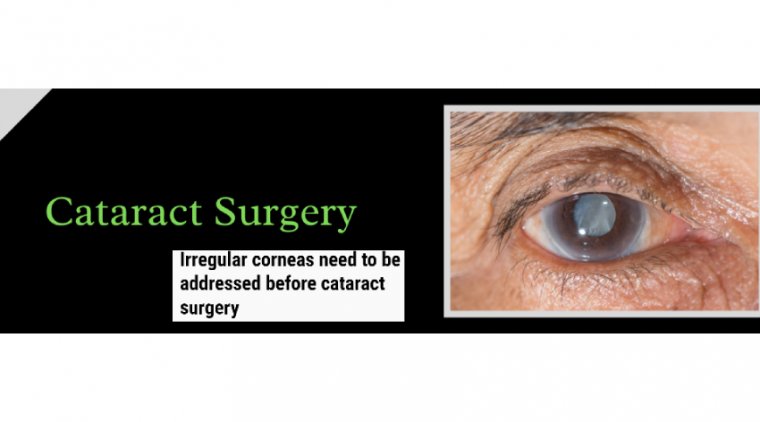
Cataract Surgery & Diseased Corneas
Corneal epithelial, stromal and endothelial disease may impact and be impacted by cataract surgery.
Appropriate perioperative management and advances in surgical techniques and technologies allow for successful cataract surgery in patients with corneal disease. Signs of corneal disease should be identified preoperatively to allow for surgical planning and optimal visual outcomes.
Cataract surgery has the potential to contribute to the progression of preexisting corneal diseases; similarly, corneal disease can limit surgical techniques and even prevent well tolerated cataract extraction.
Ensuring optimal visual outcomes requires the identification of preoperative corneal disease and risk factors for postoperative corneal complications.
Management may include perioperative use of pharmacotherapy or alternative surgical techniques, and in cases of more severe corneal disease, may include simultaneous or staged keratoplasty.
Different corneal diseases present different challenges to achieving the desired refractive outcome after cataract surgery.
Achieving the desired refractive outcome after cataract surgery can be a challenge in eyes with corneal disease. However, excellent results are possible with careful preoperative evaluation coupled with attention to IOL selection and surgical technique.
These are special situations that often require a change in technique. Performing cataract surgery in eyes with irregular astigmatism, Fuchs corneal endothelial dystrophy/endothelial compromise, keratoconus, a history of penetrating keratoplasty (PK), and prior keratorefractive surgery is important for surgeons to tailor their approach to the circumstance and not use a cookie-cutter approach for all procedures.
A question that cornea specialists often hear from their anterior segment colleagues is “How do I modify my approach to cataract surgery in an eye with an abnormal cornea?”
In this first installment of a three-part series, Kavitha R. Sivaraman, MD, at the Cincinnati Eye Institute, hosts a discussion with Nicole R. Fram, MD, at Advanced Vision Care in Los Angeles, and Joshua C. Teichman, MD, MPH, at Prism Eye Institute and the University of Toronto.
The trio share their pearls for cataract surgery in the context of autoimmune-related dry eye and endothelial dysfunction.
Pre-Op Prep in Severe Dry Eye
Dr. Sivaraman: For a patient with severe autoimmune-type dry eye—such as Sjögren’s syndrome, graft-vs.-host disease (GVHD), or mucus membrane pemphigoid—what do you look for preoperatively, and what are your criteria for offering cataract surgery?
Dr. Fram: Cataract surgery in these patients can be challenging. For a good outcome, you need to make sure that the ocular surface is healthy enough for measurements so that you can get reliable topography and biometry results.
It’s also important to minimize inflammation preoperatively, whether that’s with an antiinflammatory, like cyclosporine or lifitegrast, preservative-free dexamethasone, or even serum tears.
You should have a conversation about how to use these medications properly. Serum tears, for example, contain no preservatives, so you should discuss with the patient the importance of using a refrigerated fresh bottle every three to seven days and making sure to store the remainder in the freezer.
The patient’s disease stage dictates my preoperative plan. If the patient has early stage Sjögren’s syndrome or very early mucous membrane pemphigoid, I focus on the eyelids, making sure that there is no keratinization of the lid margins.
If there is keratinization of the eyelids, there is typically overgrowth of bacteria, which increases endophthalmitis risk. Before cataract surgery, I pretreat every patient with a hypochlorous acid antiseptic, which is particularly important in autoimmune dry eye.
If the dry eye is relatively mild, the patient may be a candidate for a toric IOL, an extended depth of focus lens, or an enhanced monofocal IOL.
However, if the dry eye is severe and the cornea is irregular despite aggressive preoperative treatment, it is best to place a traditional monofocal lens for the most reliable outcome.
Managing Autoimmune Dry Eye During Cataract Surgery
Dr. Sivaraman: What are some perioperative pearls for cataract surgery in the setting of autoimmune dry eye?
Dr. Fram: Intraoperatively, I coat the eye with methylcellulose or OcuCoat (Bausch + Lomb) to keep the surface moist because an epithelial defect can be particularly problematic when corneal dysfunction is present.
After the procedure, I place a disposable soft contact lens or a bandage contact lens; I’ve found that this helps avoid many complications that are common in the early post-op period.
It’s crucial that you maintain the eye with the same intensity postoperatively as you did preoperatively.
For my patients with GVHD or mucous membrane pemphigoid, I may give a subconjunctival injection of triamcinolone or dexamethasone to help them through the perioperative period.
Dr. Teichman: When treating mucus membrane pemphigoid in particular, you need to be extremely cautious to avoid injuring the conjunctiva. As much as possible, I try to avoid touching the conjunctiva, and I anchor the eye with a dry Weck-Cel sponge (Beaver-Visitec).
Dr. Sivaraman: I tend to avoid topical NSAIDs preoperatively because epithelial toxicity can occur in autoimmune-type dry eye, even from a once-daily NSAID formulation.
The rare cases that I have seen of NSAIDrelated melts have been in patients with autoimmune disease, such as in rheumatoid arthritis and Sjögren’s syndrome.
I sometimes prescribe a preservativefree formulation of methylprednisolone, dexamethasone, or Lotemax ointment (Bausch + Lomb) because autoimmune dry eye patients patients tend to be very sensitive to benzalkonium chloride and other preservatives.
And I learned a trick from one of my partners: At the end of the case, any unused dispersive viscoelastic is placed on the cornea and the eye is patched shut for a couple of hours.
It seems that the effect is like that of a bandage contact lens—letting the eye recover from the betadine and preserved drops that you use perioperatively.
Challenging Corneal Topographies
Dr. Sivaraman: Despite our best efforts to optimize the ocular surface, sometimes the corneal topography is still problematic. Maybe the eye is still very dry, even though it’s not actively inflamed.
Maybe the patient is dependent on a scleral lens or bandage contact lens but visual acuity is 20/ 400 from a posterior subcapsular cataract, and it’s time to choose an IOL. What is your approach?
Dr. Teichman: I do my best to get the contact lenses out for as long as possible. In theory, a properly placed scleral lens should vault completely over the cornea and shouldn’t need to be out for very long, but you’re making an assumption in trusting that the fit is good.
When it comes to soft contact lenses, ophthalmologists vary in how long they want patients to have them out preoperatively—from as little as three days to as long as two weeks.
Once the patient is ready for testing, the first thing I look at are the mires on topography, to get an idea of corneal curvature and the quality of the scan, and I look for consistency among multiple test results over time.
You want to have reliable results in terms of all your measurements: biometry, refraction, keratometry, topography, and tomography. Tomography is less influenced by tear film than topography, so tomography can be more informative than topography for dry eye/ocular surface patients.
Surgical Evaluation in Endothelial Disease
Dr. Sivaraman: What is your approach to the initial evaluation and IOL selection for cataract surgery in eyes with endothelial disease?
Dr. Sivaraman: For example, maybe the case involves Fuchs dystrophy, posterior polymorphous corneal dystrophy, or prior glaucoma surgery, and the endothelium is known to be compromised.
I’m always thinking about whether the patient is likely to need endothelial keratoplasty (EK) in their lifetime.
Some cases have obvious edema, so you know that a graft or maybe a Descemet-stripping only procedure will be needed. The trickier cases are those patients with a compact stroma who have advanced endothelial disease because you need to predict if manifest edema is likely to occur after cataract surgery.
I like to think that I’m getting better the longer I do this, but you can’t always predict which eyes will decompensate. Characteristics that I consider are patient age and cataract density, especially in the context of a shallow anterior chamber.
I tend to perform specular microscopy on these patients to check the endothelial mosaic preoperatively, but it’s not just about the cell count; the cell morphology and functional status also matter.
An endothelial cell count over, say, 1,000 can bring a false sense of security, and some surgeons use criteria of cell count 640 µm as an indication for a triple procedure (i.e., phacoemulsification, IOL implantation, and endothelial transplantation).
I don’t find pachymetry results at a single point in time to be particularly useful. Without longitudinal data, you can’t know if corneal thickness of, say, 610 µm is normal for that person; if, for example, the native cornea is 480 µm, that would indicate gross edema.
Dr. Teichman: I agree. Surgical planning based on cell count and pachymetry results is dated for many reasons. First, phaco technology has significantly improved (with corneas that may have previously decompensated now doing very well).
Second, the study we are all referring to was when cataract surgery was to be combined with penetrating keratoplasty, which has a poorer risk/ benefit profile than EK.
So both cataract and cornea surgery have really improved. Pre-op planning involves determining whether the visual problem stems from the endothelial disease, the cataract, or both.
The first question I always ask patients is “Do you have blurry vision in the morning?” I think that morning edema, on its own, is an indication for EK nowadays. I avoid hydrophilic acrylic IOLs in endothelial disease.
If the patient needs EK in the future, the procedure’s air bubble can calcify these lenses. Additionally, if I think the eye is at risk of decompensating, I typically aim to keep the patient a little myopic during the cataract surgery.
If I’m doing a Descemet’s membrane endothelial keratoplasty (DMEK), I usually aim for somewhere between –0.5 D and –1.0 D.
If you’re performing the cataract surgery but would be referring the patient to a cornea specialist should decompensation occur, you want to account for the hyperopic shift that your local cornea specialist would have after EK.
Another consideration is astigmatism and toric lenses. If there’s corneal edema, the astigmatism can increase, decrease, and/or change axes in response to DMEK.
As an unofficial rule, if the eye has more than 2 D of cylinder, it is probably indicative of inherent cylinder in the cornea.
If the patient likely will require DMEK, I’m happy to place a toric lens in these cases, as I know it should help to debulk the astigmatism, even if it does not eliminate it completely.
I generally avoid toric lenses for eyes with 1.5 D or less of cylinder in the context of edema, as the results are more variable.
Dr. Sivaraman: I agree, especially for against-the-rule astigmatism. I may consider a toric lens if the pattern is regular and the eye has more than 2 D of cylinder, but I stipulate that the patient should still expect to wear glasses, albeit hopefully a lowerstrength prescription.
Dr. Fram: I will also consider a toric IOL if I have reliable data on the patient over time, the pachymetry results show that the cornea has stable thickness (e.g., 2.5 D of astigmatism that is not re producible on multiple measurements, I typically offer a phakic DMEK.
This technique is relatively simple to perform, provides information on the corneal shape, and expands the range of future therapeutic options.
One caveat: It is critical to use air in phakic DMEK—not gas—and before surgery, you should perform a laser peripheral iridotomy (LPI) in the office if possible.
If the cornea is so edematous that the iris features are not visible, the surgeon can perform a surgical peripheral iridotomy using a 23-gauge vitrector with an I/A cut setting, >700 mm Hg vacuum, and cut rate of 50-100 cuts/minute.
Dr. Sivaraman: It’s not just the astigmatism that’s labile in these operations; the spherical equivalent can shift as well because of post-DMEK corneal deturgescence.
If a patient undergoes implantation with a costly premium IOL and still needs a +3 D spherical prescription postsurgically, you may be thrilled that the astigmatism is corrected, but the benefit to the patient is less apparent.
The possible need for corrective lenses is worth including in the informed consent discussion preoperatively.
Dr. Fram: You bring up an important point! The thicker the cornea is preoperatively, the more likely that you will get a hyperopic result. I tend to aim even more myopic in these cases.
For example, I typically aim –0.50 D for DMEK triple and –1.25 D for DSEK triple for a distance result. In the case of an edematous cornea (>700 µm) and DMEK triple, I will aim –1.00 D to achieve a plano result.
In general, less-complicated cases (e.g., Fuchs, early pseudophakic bullous keratopathy) are managed with DMEK. More complex eyes receive nanothin DSEK which is easier to perform in postvitrectomized eyes or eyes with multiple filtering tubes.
I will even aim as high as –1.50 D to achieve a plano result in these complex eyes.
Dr. Sivaraman: I do, too.
Endothelial Disease and Cataract Surgery - Post-Op Care
Dr. Sivaraman: For post-op care in patients with endothelial disease, I often taper the steroid more slowly. What is your approach for a tenuous endothelium?
Dr. Fram: I augment the steroid intensity in the early post-op period. I give these patients Durezol (Novartis) or prednisolone acetate, and I focus on the counseling. I tell patients that it’s normal to have blurry vision in the first two weeks.
If I see deep folds and edema centrally and clearing of the periphery, I can be confident in telling the patient that vision will be clear.
If the edema and folds are wall to wall, I have a conversation with the patient about options and what to expect. In such cases, I prescribe Muro drops (Bausch + Lomb) and ointments to give the endothelium a break.
Even if the eye hasn’t improved by, say, the three-month mark, I don’t give ripasudil or netarsudil as a last-ditch wound-healing effort as I have found these eyes to be more susceptible to honeycomb keratopathy.
Dr. Teichman: Sometimes as cornea specialists, we forget that not every surgeon is as comfortable with corneal edema as we are. It’s worth revisiting the basics. When trying to determine the cause of edema postoperatively, keep in mind that there are risk factors besides endothelial damage.
For instance, patients with Fuchs dystrophy are more likely to have a Descemet’s detachment, but you may not be able to see the detachment through a cloudy cornea. It might be apparent only with anterior segment OCT.
I’ve had very good surgeons refer cases of corneal edema to me. On gonioscopy, I find a retained lens fragment, or viral keratitis, or a haptic out of the capsular bag causing an IOP spike.
With a thorough examination, you might find other causes of edema that are treatable without EK.
Dr. Sivaraman: Yes, retained lens fragments can be an issue. When the lens is dense, I’m liberal with the dispersive viscoelastic; I’ll apply it periodically during nucleus disassembly if the cumulative dissipated energy (CDE) is climbing.
However, this increases the risk of a trapped lens fragment. Keep careful track of what you’re phacoing. When you’re trying to salvage the endothelium and spare the patient a graft, the last thing you want is a retained lens fragment in the angle.